제품
Ceftriaxone Sodium
| Chemical Name | : Ceftriaxone Sodium |
| Category | : β-Lactam Antibiotics |
| Specification | : |
| HS Code | : 29419055.00 |
- 전화: +86-532-83876123
- 팩스: +86-532-83876157
- 이메일: dennis@qingmeibio.com
- 온라인: dennis10221
화학성질
| Structure Formula | : 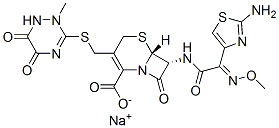 |
| CAS Number | : 74578-69-1 |
| Molecular Formula | : C18H18N8O7S3 |
| Usage | : For meningitis, pneumonia, skin and soft tissue infection, peritonitis sensitive bacteria infection, urinary system infection, gonorrhea, biliary infection, surgical trauma, sepsis and genital infection. Has been used as a first-line drug treatment of gonorrhea. |
기술매개 변수
| Items | Standard |
|---|---|
| Appearance | A white or almost white, crystalline powder |
| Solubility | This product is soluble in water, slightly soluble in methanol, in chloroform or ether several.Almost insoluble. |
| Identification | Accordant with IR spectrum obtained with reference standard |
| Appearance of solution | |
| Related substances | |
| Impurity B | ≤ppm |
| Impurity P | ≤0.0% |
| Impurity C | ≤0.0% |
| Impurity N | ≤0.0% |
| Impurity O | ≤0.0% |
| Impurity I | ≤0.0% |
| Impurity J | ≤0.0% |
| Impurity F | ≤0.1% |
| Impurity A | ≤0.0% |
| Impurity K | ≤0.0% |
| Impurity G | ≤0.0% |
| Any other impurity | ≤0.0% |
| Total impurities | ≤0.% |
| Water | 8.0%~11.0% |
| Sulphated Ash | ≤0.% |
| Residual Solvent | ≤0.3% |
| Toluene | ≤ppm |
| Assay (anhydrous basis,content of Cefadroxil) | ≥84.0% |
문서지지
The available documents are:
Chinese GMP
CEP
DMF/CTD format
The available registered countries are:
Algeria
Spain
Australia
Chinese GMP
CEP
DMF/CTD format
The available registered countries are:
Algeria
Spain
Australia