제품
Sodium alginate
| Chemical Name | : Sodium alginate |
| Category | : Pharmaceutical excipients, suspending agents and release blockers |
| Specification | : USP,EP |
| HS Code | : 3913.1000.00 |
- 전화: +86-532-83876123
- 팩스: +86-532-83876157
- 이메일: dennis@qingmeibio.com
- 온라인: dennis10221
화학성질
| Structure Formula | : 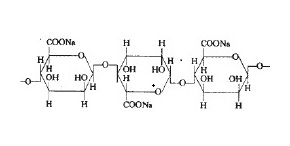 |
| CAS Number | : 9005-38-3 |
| Molecular Formula | : C5H7O4COONa |
| Usage | : Polymeric materials, sodium alginate is suspending, thickening, emulsifying, bonding and other effects, in the agent is mainly used for suspending agent, emulsifier, viscous agent, microcapsules encapsulating material and so on. As tablet binder (1% ~ 3%) and disintegrating agent (2.5% ~ 10%); used as a diluent in capsule; also used for the preparation of oral sustained release preparation, because it can delay the drug from the water soluble suspension of release; in the paste, ointment and the gel is widely used as a thickening agent and suspending agent (5% ~ 10%), and as a stabilizer in oil in water emulsions (1% ~ 3%) use. 0.5% ~ 2.5% aqueous solution can be used to smooth the skin erosion surface, can make the water of secretions and drying. Food additives: film-forming agent, emulsifier, thickener. Increased blood volume and maintain blood pressure, eliminate burn produced by histamine toxoid and trauma hemorrhage, operation before and after the cycle of system stability, a lot of hemorrhagic shock, burn shock, fever and other systemic acute dysentery dehydration, good treatment effect. Also has makes cholesterol excreted, inhibition of Pb, Cd, Sr is absorbed by the human body and the protection of the gastrointestinal tract, intestines, lose weight, the effect of reducing blood sugar. The medicine is mainly used as a suspending agent, emulsifier, viscous agent, microcapsules encapsulating material and so on. |
기술매개 변수
| Items | Standard |
|---|---|
| Appearance | White or brown-pale yellow powder |
| Identification | Test A complies |
| Test B complies | |
| Solubility | Slowly soluble in water, forming a viscous solution and colloidal, practically insoluble in ethanol 96% |
| Appearance of the solution | The test solution is more intensely colored than reference solution |
| Impurities | Heavy metals 20PPM Arsenic 1.5PPM max Lead 10PPM max Chlorides 1.0% max Calcium 1.5% max |
| Assay(dry basis) | 90.8-106.0% |
| Viscosity (1% solution on natural basis) MPA.S | 10max |
| Particle size mesh | 60 mesh |
| Loss on drying at 105° C For 4 H | 15%max |
| PH | 6.4-8.0 |
| Sulphated ash | 30%-36% |
| Calcium content | 0.3% max |
| Microbial enumeration tests | Total plate count 1000CFU/G max Yeast and mould 100CFU/G max E.COLI absent in 25G Salmonella absent in 25G |
| Conclusion | USP& EP |
문서지지
The available documents are:
Chinese GMP
CEP
DMF/CTD format
The available registered countries are:
Algeria
Spain
Australia
Chinese GMP
CEP
DMF/CTD format
The available registered countries are:
Algeria
Spain
Australia